The Coflex® Interlaminar Stabilization® Technology is an interlaminar functionally dynamic implant designed to impart a stabilization effect at the operative level(s) after a decompression surgery while retaining natural motion. It consists of a single, U-shaped component, fabricated from medical grade titanium alloy (Ti6Al4V, per ASTM F136 and ISO 5832-3). In clinical use, the “U” is positioned horizontally, with its apex oriented anteriorly and the two long arms of the “U” paralleling the long axis of the spinal processes. The implant bears load on interlaminar bone, and compresses upon spinal extension. The bone-facing surfaces are ridged to provide resistance to migration.
The Coflex® device is an alternative to fusion for a subset of lumbar spinal stenosis patients
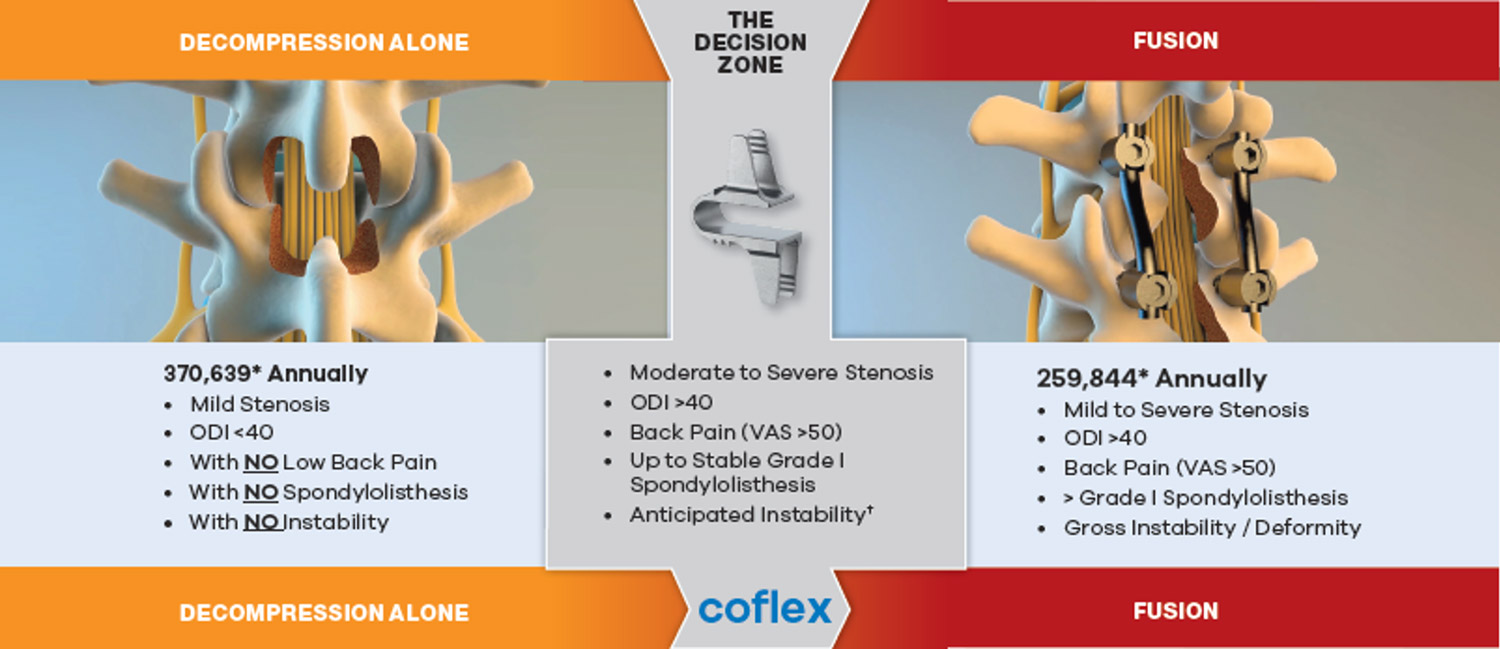
The Coflex® device is an option for those in the “decision zone” between decompression alone and fusion procedures. It is for patients that experience moderate to severe stenosis, have an ODI greater than 40 as well as a back-pain VAS score greater than 50. It is ideal for patients who have up to stable grade I spondylolisthesis. When combined with decompression, it is strong enough to support the patient’s spine for longer, but flexible enough to allow for maximum movement. It also requires minimal equipment, has a shorter recovery time, and yields effective results over time.1
The design and location of the device in the patient’s back helps to maintain foraminal height and off-loads facets to reduce leg and back pain.
What does the Coflex® device offer your patients?
The Coflex® device is the exclusive posterior lumbar motion preservation solution with proven long-term outcomes for durable pain relief and stability. It is an option for patients that do not want to go through pedicle screw fusion, but a decompression alone would not offer needed stability.

The Coflex® Device Has Been Implanted More Than 175,000 Times in Over 60 Countries, by Over 1,500 Spine Surgeons.
T Post-operative.
*Based on IMS Data, 2016.
1 “Evaluation of decompression and Interlaminar Stabilization compared with decompression and fusion for the treatment of lumbar spinal stenosis 5-year follow-up of a prospective, randomized, controlled trial” Musacchio, M., International Journal of Spine Surgery, 2016.


