The Coflex® Interlaminar Stabilization® device is the only lumbar spinal device with Level 1 evidence in two separate clinical studies. The Coflex® device has been implanted in more than 175,000 patients in over 60 countries. Many patients experience relief of their leg and back pain, return to their physical function, and were satisfied with their overall results. The following is a list of peer-reviewed studies and articles demonstrating the efficacy of the Coflex® device.
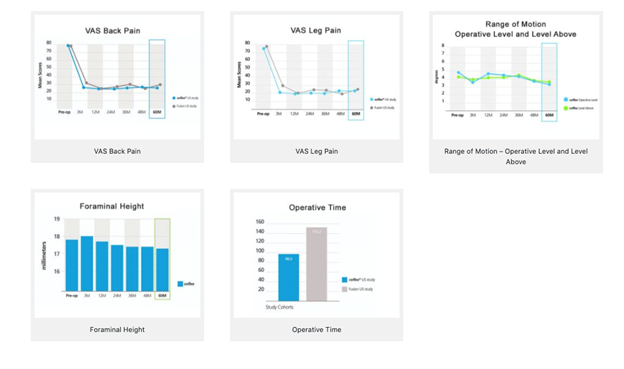
U.S. FDA Trial Overview
The objective of this study was to test the safety of the Coflex® Interlaminar Stabilization® device in patients with moderate to severe spinal stenosis.
The Study
- 322 patients (215 Coflex®, 107 fusion)
21 investigational locations
Follow up visits at 3, 12, 24, 26, 48, and 60 months
Conclusion
The Coflex® device proved to be an effective alternative when compared to pedicle screw fusion after 5 years. Results demonstrated that patients with the Coflex® device performed just as well or better in all measurements.
ESCADA Trial Overview
The Coflex® treatment was compared to decompression in the ESCADA trial to see if it would be an improved method for patients with moderate to severe spinal stenosis.
The Study
- 230 patients (115 Coflex®, 115 decompression alone)
7 investigational locations
Follow up visits at 3, 12, and 24 months
Conclusion
The Coflex® device statistically outperformed decompression alone for Composite Clinical Success*. The results were tangible:
Increase in walking distance
Decrease in pain management
Maintained foraminal height
Extended the lifetime of the decompression procedure to 2 years
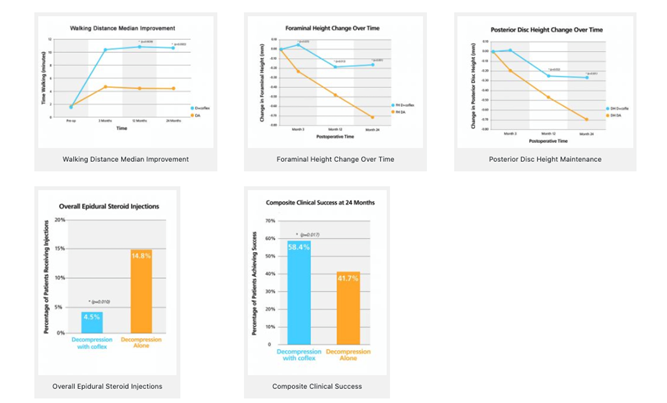
*CCS: Criteria where overall trial success was based on a composite endpoint; a combined outcome measure in which all four components must be met: 1) disability score improvement of at least 15 points; 2) no reoperations or subsequent lumbar injections; 3) neurological maintenance or improvement without worsening; and 4) no device- or procedure-related severe adverse events.
- ESCADA 2 Year Results
- 5 Year U.S. Study Results
- 2 U.S. Year Study Results
- 2 U.S. Year Study Results – SPONDY COHORT
- Determination of the In Vivo Posterior Loading Environment of the Coflex® Interlaminar-Interspinous Implant
- Role of the Coflex® device as an Adjunct to Decompression for Symptomatic Lumbar Spinal Stenosis
- Perioperative Outcomes, Complications, and Costs Associated with Lumbar Spinal Fusion in Older Patients with Spinal Stenosis and Spondylolisthesis


